How true is it that atoms are immortal (and if so, why does every living thing die)?
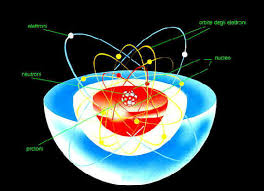
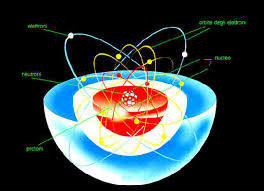
All matter, from the clouds in the sky to the stars in the firmament and the water you see flowing, is composed of atoms. Currently, every atom is composed of two fundamental parts: "An electron cloud and a nucleus, which houses protons and neutrons." "So they don't have to remain the same." Protons are capable of transforming into neutrons and vice versa, which is important because their delicate balance is what gives atoms their individual properties. When the number of neutrons or protons changes, the type of atom is transformed, meaning it becomes a different element. "One example is potassium, found in bananas. If it decays, it transforms into calcium, a completely different element." All matter, from the clouds in the sky to the stars in the firmament and the water you see flowing, is composed of atoms. Currently, each atom is composed of two fundamental parts. "An electron cloud and a nucleus, which houses protons and neutrons." "So they don't have to remain the same." Protons are capable of transforming into neutrons and vice versa, which is important because their fragile balance is what gives atoms their individual properties. When the number of neutrons or protons changes, the type of atom transforms, meaning it becomes a different element. "One of the things we do is collide lead ions at very high energy. What happens is that they are converted almost entirely into pure energy, and then they decay into a large number of fragments," he says. "In these collisions, the temperature generated is extremely high, reaching 100,000 times that of the Sun's core. And at those temperatures, the core melts, leaving you with a liquid composed primarily of gluons and quarks." Gluons and quarks are very small particles that combine to form neutrons and protons in the nucleus of an atom. By causing two atoms to collide, you obtain what specialists call a quark-gluon plasma.
11/28/20251 min read
Contenido de mi publicación
